Fuels play a significant role in combustion industries, energy, and heat supply. These vital resources are used in various industrial processes for heat generation, combustion, and mechanical energy supply. Among them, burner fuel is crucial for efficient and controlled combustion in industrial burners, ensuring optimal heat output and energy utilization. The role of fuel types extends beyond energy provision; their environmental and economic impacts, including the efficiency and emissions of burner fuel, are of great global significance.
Choosing the right fuel type (burner fuel) impacts not only the efficiency and effectiveness of equipment but also operational expenses, the depletion of natural resources, and pollutant emissions. The selection process of fuel type plays a crucial role in balancing energy efficiency and environmental impact, influencing sustainability efforts across various industries.
There are three primary groups of fuel types according to their physical form: gas, liquid, and solid, each with its own characteristics, advantages, and challenges. This article will provide a comprehensive review of the various fuel types used in industrial burners and analyze the features of each category.
Definition of Fuel
To initiate and sustain the combustion process, three essential elements must be present, commonly referred to as the fire triangle. fuel, oxygen, and an initial energy source to start the reaction. Fuel includes flammable substances such as coal, oil, and gas, primarily used to ignite and sustain combustion for heat generation.

Fuel Heating Value
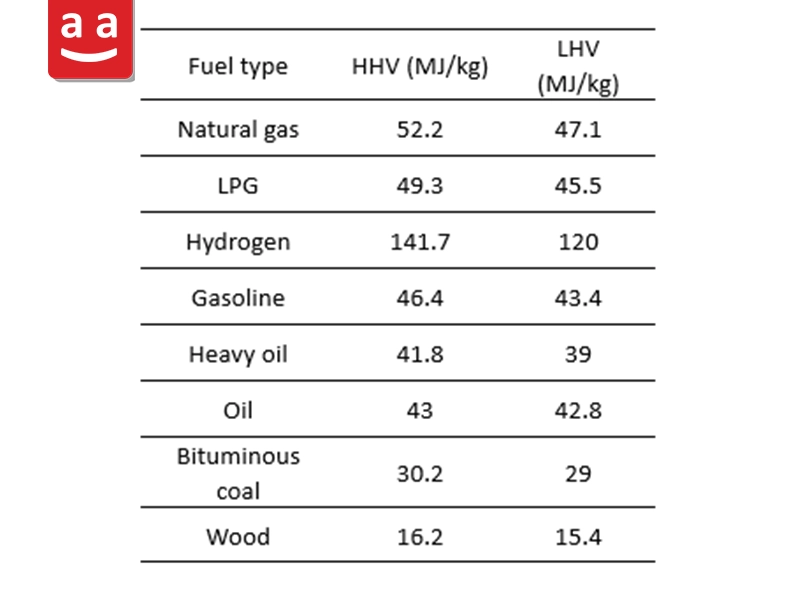
It indicates the amount of heat released during the complete combustion of a unit mass of fuel. Specifically, when one kilogram of fuel undergoes full combustion, the amount of heat emitted is referred to as its heating value. The heating value of fuels is defined in two ways: Lower Heating Value (LHV) and Higher Heating Value (HHV). The distinction between these two values depends on the physical state of water in the combustion products.
Higher Heating Value (HHV)
In this case, it is assumed that the water vapor produced during combustion condenses into liquid form. Therefore, the latent heat of vaporization of the water is also included in the calculation of the energy released from combustion. In simpler terms, HHV represents the maximum chemical energy obtainable from a unit mass of fuel, as it incorporates the heat released when the vapor condenses into liquid water.
Lower Heating Value (LHV)
Here, the water present in the combustion products remains in vapor form, and condensation does not occur. As a result, the latent heat of vaporization of the water is not recovered and is lost from the system. As a result, LHV is lower than HHV. LHV is considered a more common measure in many industrial applications where combustion products are discharged at high temperatures and contain water vapor.
Classification of Fuels
Fuels are classified into three types: gas, liquid, and solid, according to their physical state. The selection of fuel impacts both system performance and emissions. Gas fuels have lower sulfur pollutants and are easier to combust. Liquid fuels require specific equipment for atomization and can emit pollutants if combustion is incomplete. Solid fuels, like coal, produce substantial emissions and have fewer applications. Understanding the specific characteristics of each fuel is vital for choosing the best option for a particular use and achieving the highest efficiency.
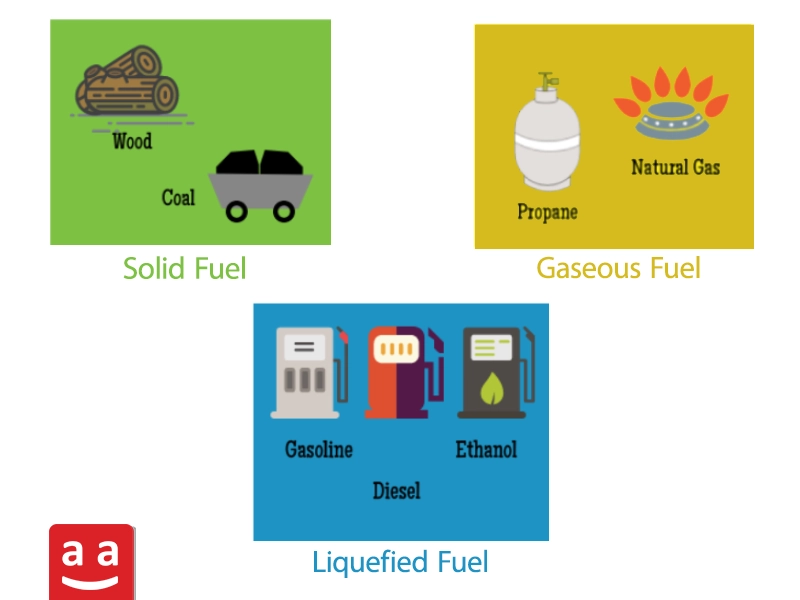
Types of Gaseous Fuels
Gaseous fuels come in various types, with the most important ones being: natural gas, hydrogen, liquefied petroleum gas (LPG), and process fuels.
Natural Gas
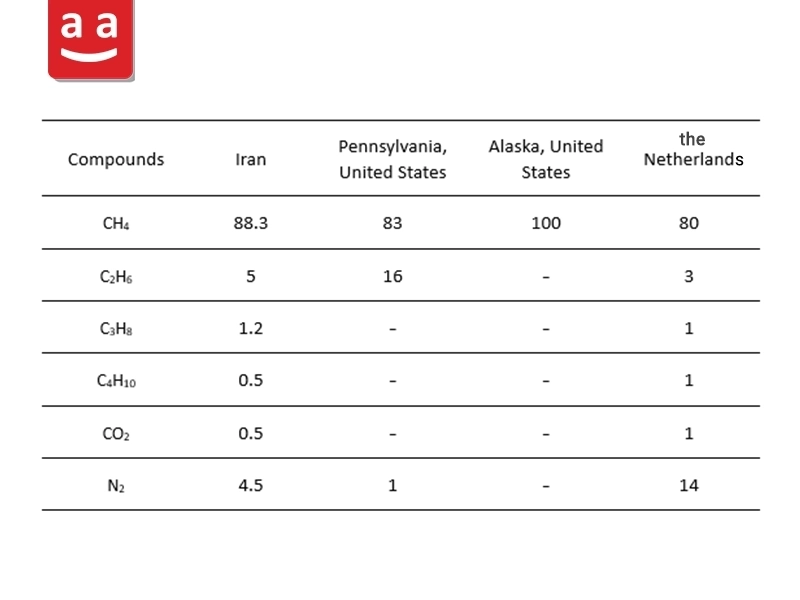
Natural gas, a fossil fuel, is primarily made up of methane and is typically found near crude oil deposits. In addition to methane, this gas may contain ethane, propane, butane, and impurities such as carbon dioxide and nitrogen. The lower heating value of natural gas is about 47 MJ/kg, although this value can vary depending on its specific composition in different gas fields. The table below presents four different natural gas compositions from various locations worldwide.
Key Benefits of Natural Gas
- Lower Environmental Impact: Natural gas emits less carbon dioxide and environmental pollutants than liquid fuels and coal, making it a cleaner fuel option.
- Convenient Transport: With the widespread pipeline infrastructure, transporting natural gas is easier and more economical than other fossil fuels.
- Extensive Usage: Natural gas can be utilized in a wide array of applications, including power generation, various industries, and home heating.
- Simpler Combustion Control: Natural gas combustion is easier to control than that of liquid and solid fuels, leading to higher efficiency.
Given these features, natural gas has gained a significant share of the energy market in recent decades, and due to its greater environmental compatibility, many countries are expanding the necessary infrastructure to maximize its usage.

Liquefied Petroleum Gas (LPG)
Liquefied Petroleum Gas (LPG) is primarily a mixture of propane and butane. This fuel is used in areas without access to natural gas pipelines due to its ability to be stored in liquid form. Though it is in a gas state at standard conditions, it turns into a liquid under pressure (typically 8 bar). The Lower Heating Value (LHV) of LPG is around 45.5 MJ/kg, and its use in industries requires specific equipment, such as LPG evaporators in high-capacity applications to ensure proper fuel flow and prevent freezing of equipment.

Key Benefits of LPG
While these benefits exist, the risk of leakage and explosion remains if safety protocols are not adhered to. Hence, careful supervision of the transportation and storage of LPG is crucial to avoid possible risks.
- Easy to transport and store in cylinders or pressurized tanks.
- Reduced pollutant emissions compared to heavy liquid fuels and gasoline.
- Flexibility in application: suitable for both domestic and industrial use.
Hydrogen
Hydrogen is a clean and energy-dense fuel that generates only water vapor during combustion and does not release any greenhouse gases. This makes it a suitable choice for reducing greenhouse gas emissions.
Hydrogen is divided into three main types based on its production method: gray hydrogen, blue hydrogen, and green hydrogen. Gray hydrogen is derived from natural gas or coal and is accompanied by CO₂ emissions. Blue hydrogen follows a similar production process but reduces carbon emissions through carbon capture and storage. In contrast, green hydrogen, produced via water electrolysis using renewable energy, is completely carbon-free and has the highest environmental compatibility.
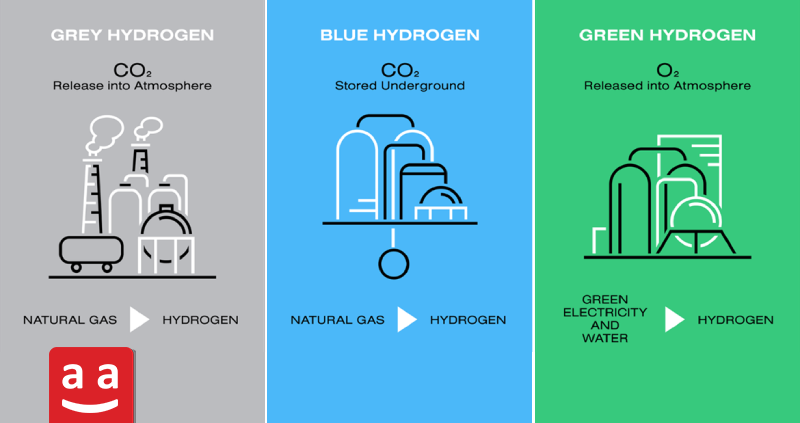
Despite the high potential of hydrogen as a fuel for the future, its development and expansion require dedicated infrastructure. Major challenges include distribution networks, fueling stations, secure storage systems, and cost-efficient production technologies. Investment in this field and technological advancements can pave the way for a hydrogen-based economy, playing a crucial role in the transition to clean energy and reducing dependence on fossil fuels.
Process Fuels
Process fuels are mainly obtained in refining and petrochemical plants and are a mix of hydrogen, carbon monoxide, carbon dioxide, methane, and other by-product gases. In processes like natural gas reforming and methanol production, part of the produced gas (synthesis gas) is directly used in the main reaction, but some of it remains. This extra gas contains valuable compounds that are either flared into the atmosphere or returned as fuel to furnaces and boilers to extract usable energy. This method improves process efficiency and reduces pollution.

Key Benefits of Process Gas Fuels
- Energy recovery and efficiency improvement: Preventing the loss of energy present in process gas.
- Pollution reduction: By reducing fuel wastage, the emission of greenhouse gases and pollutants decreases proportionally.
- Economic savings: Generating steam or electricity from excess gases and reducing energy costs.
Gaseous Fuel Combustion
For gas fuels, since both air and fuel are in similar physical states, combustion takes place in a more efficient and complete manner. However, to ensure complete combustion with minimal emissions, the fuel and air must be mixed appropriately and accurately. In gas burners, the fuel flow is driven by gas pressure, so there is no need for extra equipment to facilitate the flow of the fuel.
Raadman gas burners, equipped with advanced design and optimal combustion technology, can burn a variety of gas fuels, such as natural gas, LPG, and process gases. These burners fall under NOx classes 2 and 3 for natural gas and LPG. In pre-mixed and post-mixed configurations, using innovative premix combustion technology, they also achieve NOx classes 4 and 5 with natural gas.
Liquid Fuels
Liquid fuels refer to a group of fuels that are in liquid form at standard temperature and pressure. Some of the most important liquid fuels include diesel, heavy fuel oil, and gasoline. These fuels are typically produced through the refining of crude oil and are used in various sectors, such as transportation, heating, and electricity generation.
Liquid fuels are tyipcally composed of a blend of different hydrocarbons, and their physical and chemical characteristics can change somewhat depending on the region or time of year.
Gasoline
It is one of the most well-known liquid fuels, mainly used in transportation. It is produced through the refining of crude oil and is a mixture of hydrocarbons containing 4 to 12 carbon atoms per molecule. For simplicity, its chemical formula is often considered as octane (C8H18). The density of gasoline is approximately 737 kg/m³, and its lower heating value (LHV) is 43.4 MJ/kg. However, as mentioned, these values may vary slightly depending on time and location.
Oil
Oil (also known as gas oil or diesel fuel) is used in diesel engines and in oil burners for boilers. Oil is another product derived from crude oil and is a mixture of various hydrocarbons containing 14 to 20 carbon atoms. The density of oil is generally around 840 kg/m³, and its lower heating value (LHV) is 42.8 MJ/kg, although these values may vary slightly depending on the season and location.
Heavy Oil
Mazut, or heavy oil, is another product obtained from the crude oil distillation tower. This fuel type has a high level of pollution and its use needs specific requirements. The primary application of heavy oil is in power plant boilers and some other industrial boilers.
Mazut is categorized into grades 100, 180, 280, and 380, based on its viscosity at 50°C. Due to the high viscosity of this fuel, it needs to be preheated for pumping to reduce its viscosity. Another classification criterion for heavy oil is its sulfur content, which varies between 0.5% and 3.5%. Heavy oil has a higher density compared to oil and gasoline, with a range of 890-990 kg/m³ across various grades.
Liquid Fuel Combustion
To carry out the combustion process of liquid fuels, they first undergo evaporation and then burn in a gaseous state. To increase the evaporation rate and the reaction speed, liquid fuels are atomized into fine droplets to enhance their surface area for better interaction with air and heat transfer. Liquid fuels are atomized using three methods: pressure atomization, air or steam atomization, and rotary cup atomization.
To learn more about liquefied gas combustion, refer to the article “Liquefied Petroleum Gas Combustion in Forced Draft Burners”.
Raadman burners, in the field of liquid fuels, deliver efficient and low-emission performance by leveraging advanced technologies. They utilize pressure atomization and air or steam atomization techniques to ensure complete and uniform combustion, significantly reducing pollutant emissions.

Solid fuels
Solid fuels are generally composed of organic (combustible) compounds and inorganic (non-combustible) materials. They exist in either natural or compressed forms. This category includes wood, coal, dry agricultural waste, and biomass fuels.
Coal
Coal is an organic rock that can burn, mainly composed of carbon, hydrogen, and oxygen. It is categorized into anthracite, bituminous, sub-bituminous, and lignite. Coal types differ in their heating values, which range from 15 to 35 MJ/kg. Its wide availability, low cost, and high calorific value have kept coal as one of the major sources for electricity generation, industrial heating, and as fuel for blast furnaces in the steel industry. However, environmental issues such as CO2, SO2, NOx emissions, and ash production present major challenges in its extensive use.
In an industrial burner using coal, the coal is typically pulverized first and then mixed with high-speed air. This process enhances the combustion speed and quality of the fuel, which is crucial for industrial applications.

Wood and Bio Pellets
Wood is one of the oldest fuels used by humans. The moisture content of wood is a key factor in determining its thermal value and combustion quality. Dry wood with a moisture content of 20-25% produces more heat and has a heating value of approximately 15.4 MJ/kg. It is used in biomass power plants and for rural heating, but its lower energy density compared to fossil fuels and the substantial effect of moisture on combustion efficiency remain challenges.
Wood and biomass pellets are produced by compressing wood, forage, and agricultural residues, usually with moisture content under 10%. These pellets are employed in small and medium-sized boilers for residential heating or industrial energy production.
Coke
It is a type of solid fuel that is obtained by heating coal or crude oil without the presence of air. This hard and porous substance has a high concentration of pure carbon and produces very little smoke when burned. The primary application of coke is in the steel industry, where it serves as both a fuel and a reducing agent in blast furnaces. Although its high calorific value and carbon purity are advantageous for various industries, its production process is costly and requires specialized equipment.
Key Considerations in Fuel Selection
In various industries, selecting the appropriate fuel type plays a crucial role in process efficiency, operational costs, and pollutant emissions. Several factors affecting this decision include availability, pricing, physical and chemical characteristics, pollution levels, and existing infrastructure. Additionally, adherence to safety regulations and environmental concerns plays a crucial role in fuel selection.
Accessibility and Cost
The most important criteria in choosing a fuel are ease of access and the cost of procurement and transportation. A fuel type that is abundantly available in a region is usually cheaper and does not require complex infrastructure for supply and storage.
Heating Value
Fuel types differ in in terms of energy output. The higher the heating value of burner fuel, the less volume or mass is required to produce a specific amount of energy.
Physicochemical Characteristics
To achieve optimal and safe performance, the physical and chemical attributes of fuels—such as density, viscosity, flame speed, and chemical composition—must be suitable for the combustion process, equipment, and the materials used in system components.
Emissions and Environmental Considerations
When selecting fuel (burner fuel), it is essential to account for pollutants like carbon monoxide (CO), sulfur dioxide (SO₂), nitrogen oxides (NOₓ), and particulate matter (PM). Environmental regulations may limit the use of certain fuels or require the implementation of pollutant abatement equipment. The “NOx Formation in Combustion” provides thorough insights into NOx, its environmental effects, and methods to reduce its emissions. We recommend reviewing this article.
Fuel Application and Combustion Equipment
Selecting the appropriate fuel type is influenced by the kind of combustion equipment, including furnaces, boilers, turbines, or engines. Combustion systems are typically designed for specific fuel types, and switching fuels might necessitate major adjustments or part replacements.
Safety and Maintenance Conditions
Factors such as safe storage conditions, the risk of ignition or explosion, and the requirement for leak control or pre-heating are significant in choosing fuel (burner fuel). Heavy liquid fuels and solids often require special storage and handling, while some gaseous fuels necessitate pipeline infrastructure and pressurized storage tanks.
Supply Stability and Political Issues
In numerous instances, the reliability of energy supply and minimizing dependence on imports or global fuel market changes affect fuel selection. Many countries prefer fuel types that are domestically available for energy security purposes.
Current Infrastructure
The availability of pipeline networks, transmission lines, storage facilities, and refining or specific supply units can influence the cost and availability of various fuel types.
A Complete Guide to Different Fuels
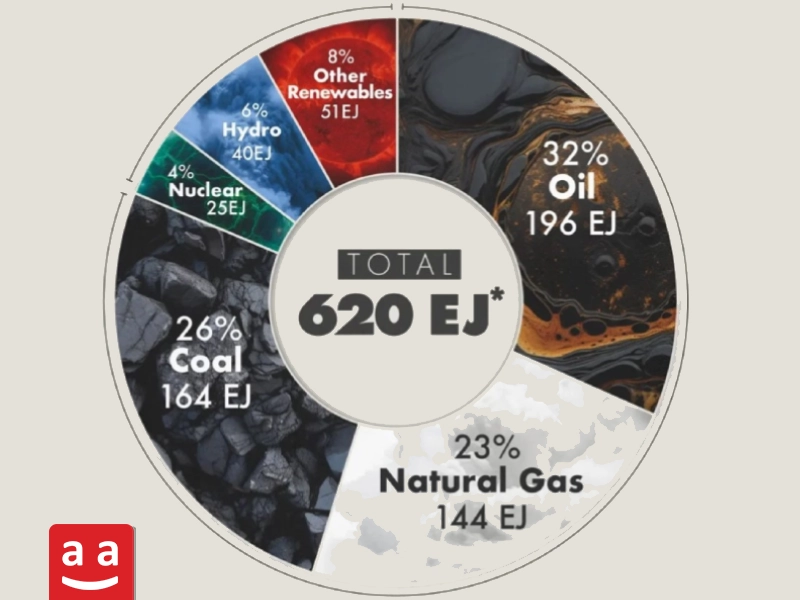
While the use of renewable energy methods for power generation is on the rise, various fuels continue to be the main source of energy in industries, building heating, and transportation. One of the key criteria for categorizing fuels is their physical state, which includes gaseous, liquid, and solid fuels. Each of these fuels has its own advantages and limitations.
Gaseous fuels are widely used due to their cleaner combustion and easier control. Liquid fuels, despite their high calorific value, produce more emissions compared to gaseous fuels and require equipment such as atomizers and sometimes pre-heating. Solid fuels are still prevalent in some industries and regions, but they generally produce more pollution, particularly coal, compared to the other two fuel types.
To learn more about gas burners, you may refer to the article “Gas Burners; main applications and key features“.
Factors like accessibility, heating value, infrastructure, environmental concerns, and cost are crucial in fuel selection. Integrating new technologies with appropriate fuel types can fulfill both industrial and environmental needs, providing an effective solution to current global challenges.
Raadman burners are designed to leverage cutting-edge technologies for combustion control and optimization. They are capable of working with different gaseous fuels as well as liquid fuels like diesel and mazut, catering to a broad spectrum of industrial applications.